Introduction
Viral diseases that pose a serious threat to society occur frequently, and preventing and treating viral infections have become major scientific problems. In particular, respiratory viruses have high infectivity and high incidence. Virus variability, drug resistance, and the high risks of drug research and development have resulted in there being only a handful of drugs for treating viral diseases.
Chinese herbs are the pharmaceutical ingredients that are collected, processed, and prepared according to the basic theory of traditional Chinese medicine (TCM), which explains the mechanism of action and guides clinical applications. Most TCMs are plant-based; thus, there is a saying that “all kinds of herbs are grass-based”. The prevention and treatment of viral diseases with TCM has a long history and clinical practice, from the Treatise on Febrile Diseases written around 2000 years ago and the Treatise on Pestilence in the Ming (1,368–1,644) and Qing (1,644–1911/12) dynasties, to the prevention and control of viral diseases in the modern era, reflects the advantages of TCM in this field (Zhang et al., 2019; Zhu et al., 2021). At the end of 2019, an outbreak of a novel coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV)-2, quickly became a pandemic. Although many countries worldwide struggled to combat the spread of the virus, China rapidly controlled the outbreak, and TCM played an important role in treating coronavirus disease 2019 (COVID-19).
According to the TCM characteristics and the characteristics and pathogenic mechanism of respiratory viral diseases, this review systematically describes the relationship among virus, host, and TCMs to provide a TCM strategy for treating respiratory viruses.
Overview of Respiratory Viruses
Morbidity and mortality due to respiratory diseases are high worldwide (Burney et al., 2015), and 90% of respiratory infections are caused by viruses, the majority of which are RNA viruses, such as orthomyxoviruses, paramyxoviruses, and coronaviruses, and rhinoviruses, and some of which are DNA viruses, such as adenoviruses. Orthomyxoviruses include influenza A virus (IAV) and influenza B virus (IBV), which are characterized by segmental RNA, variation, hemagglutination, and absence of hemolysis. Paramyxoviruses include respiratory syncytial virus (RSV), parainfluenza virus, measles virus, and mumps virus, and they have a low frequency of RNA mutation in different segments and show hemagglutination and hemolytic activity. Coronaviruses include the SARS-CoV-2, SARS-CoV, and MERS-CoV novel coronaviruses, which have high pathogenicity and variability (Battles and McLellan, 2019; Abdelrahman et al., 2020).
Respiratory viruses are highly contagious and transmitted mainly through respiratory secretions, stools, urine, droplets, air and contact (Weber and Stilianakis, 2021). Most respiratory viruses occur in seasonal outbreaks, with infants, the elderly, and immune-compromised populations at high risk (Nichols et al., 2008), and the prevalence and severity vary across geographical regions and populations (Moriyama et al., 2020). Infection often causes oral, nasal, and pharynx discomfort, airway inflammation, and lung injury, and serious cytokine storms may result in acute respiratory distress and multiple organ failure, and even lead to death of patients (Abdelrahman et al., 2020). For example, the Spanish flu, which began in 1918, killed tens of millions of people and the outbreak of the H1N1 virus in 2009 killed hundreds of thousands of people worldwide (Garten et al., 2009; Shieh et al., 2009). By June 2021, SARS-CoV-2, which was detected at the end of 2019, has infected nearly 200 million people, and killed more than 3.5 million people.
Thus, the prevention and treatment of respiratory virus diseases is a crucial global health issue. Guided by the basic theory of TCM, TCMs have unique advantages in the prevention and treatment of respiratory viruses through the overall regulation of human immune function due to its multi-component and multi-target characteristics.
Basic Theory of TCM
The basic theory of TCM has “holism” as its guiding principle and “syndrome differentiation and treatment” as its method of diagnosis and treatment, guiding the use of TCMs against viruses. The principle of holism regards the body as an organic whole, and understands the occurrence and development of local diseases as related to the whole; thus, local diseases can only be treated effectively by considering the whole body. The concepts of syndrome differentiation and treatment are defined as follows. Syndrome differentiation is the process of proving and distinguishing the type of disease, that is, knowing the location, etiology, properties, and the relationship between the “zheng (energy)” and “xie (evil)” of the disease, which reflect the nature of pathological changes. Treatment is the process of identifying the appropriate treatment methods according to the results of syndrome differentiation. This also corresponds to the "personalized treatment" in modern medicine, which is of great significance in diagnosis and treatment (Li and Xu, 2011; Ma et al., 2019).
According to TCM theory, there are two main ways that antiviral TCMs work, which are “dispelling evil” and “fu zheng”. Dispelling evil refers to the elimination of viruses, which is usually direct inhibition or killing of viruses by Chinese herbs. The mechanism of action of this kind of herb is like that of direct-acting antiviral drugs in Western medicine (direct-acting antivirals). Fu zheng refers to improving the body’s physical fitness and ability to resist evil and to rehabilitation; zheng qi is stored in the body, and prevents evil, which is also an important aspect of the antiviral mechanisms of TCMs. These two modes of action are also reflected in the mechanism of TCM treatment of respiratory viral diseases (Wang et al., 2020a).
Antiviral Research Guided by Holism and Syndrome Differentiation and Treatment
Figure 1 shows the relationship between TCM antiviral theory with respect to the basic theory of TCM and modern medicine. We discuss treatment of COVID-19 with TCM as an example of using the principles of holism and syndrome differentiation and treatment. In TCM theory, COVID-19 belongs to the category of epidemic disease. COVID-19 is caused by the invasion of the exogenous pathogen SARS-CoV-2 and the deterioration in human immune function, which exacerbates the imbalance in the body (excessive immune inflammatory reaction) and causes organ dysfunction. From the perspective of holism, COVID-19 is a struggle between the virus and the immune system, which leads to an imbalance in the homeostasis of the human internal environment. From the perspective of syndrome differentiation and treatment, the progress of the disease can be divided into the initial stage, the intermediate stage, the severe or critical stage, and the recovery stage. Different treatments target different stages of the disease; for example, clearing heat and detoxification in the early stage are part of dispelling evil in TCM. The latter three stages require the suppression of an excessive immune response and inflammation, regulation of balance in the body, and coordination of the functions of the viscera, all of which are part of fu zheng in TCM. Clinical results have shown that TCM treatment is effective for COVID-19, especially in significantly reducing the number of patients transitioning from the early and middle stages of the disease to severe and critical illness, which is key to reducing the incidence and mortality of critical illness (Lee et al., 2021).
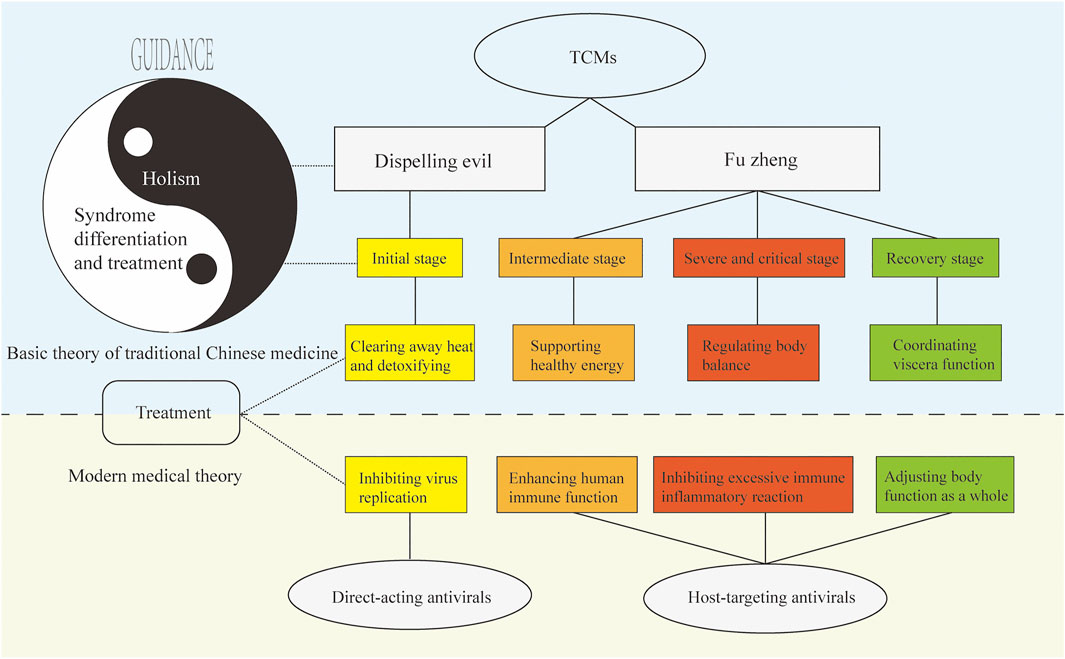
FIGURE 1. TCM antiviral theory based on the basic theory of TCM and its relationship with modern medicine. Under the guidance of “holism” and “syndrome differentiation and treatment”, TCM can remove pathogenic factors and strengthen the body, to achieve the effect of preventing and treating viral diseases, which is corresponding to the modern medical system.
Research Status of Antiviral TCMs
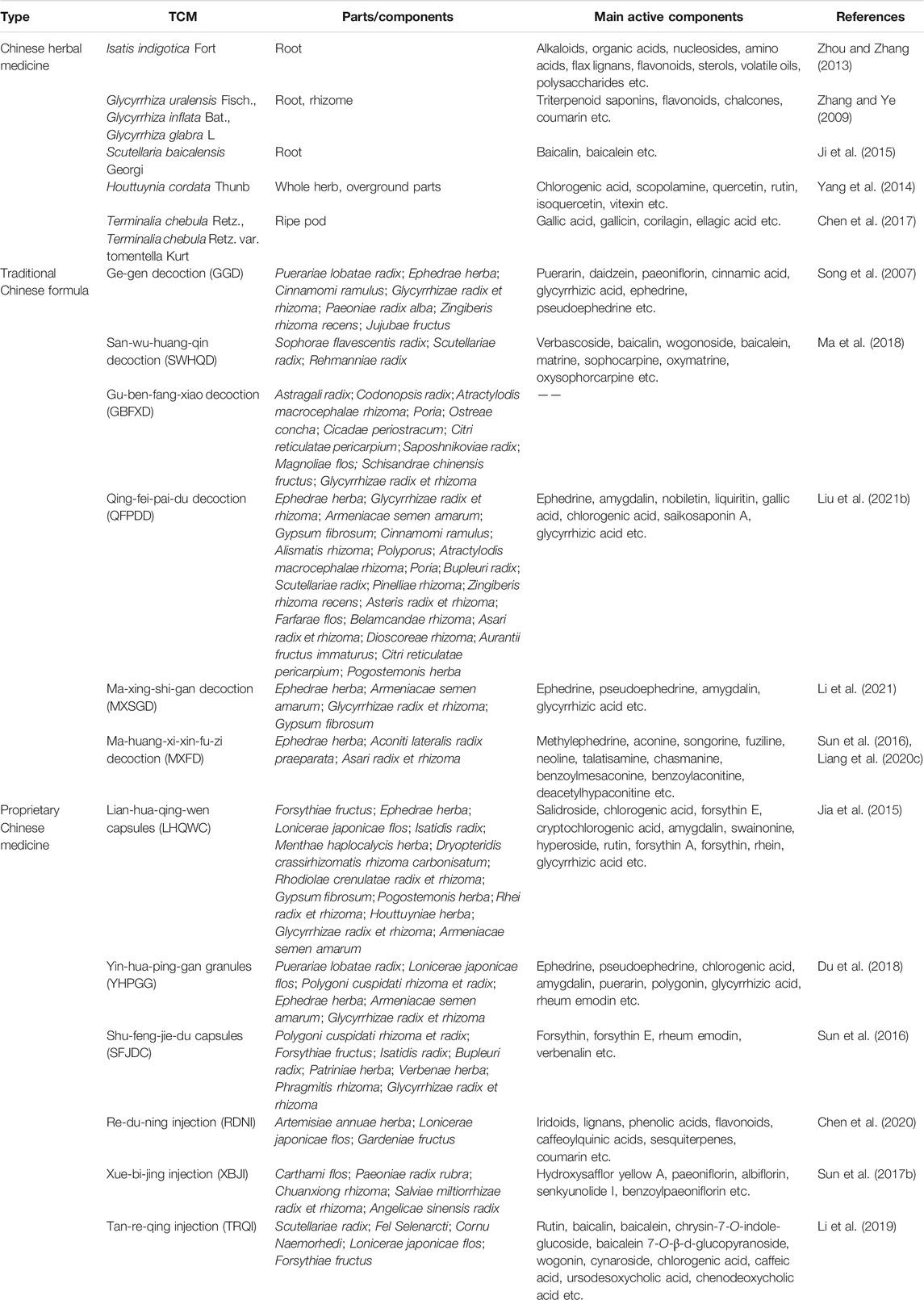
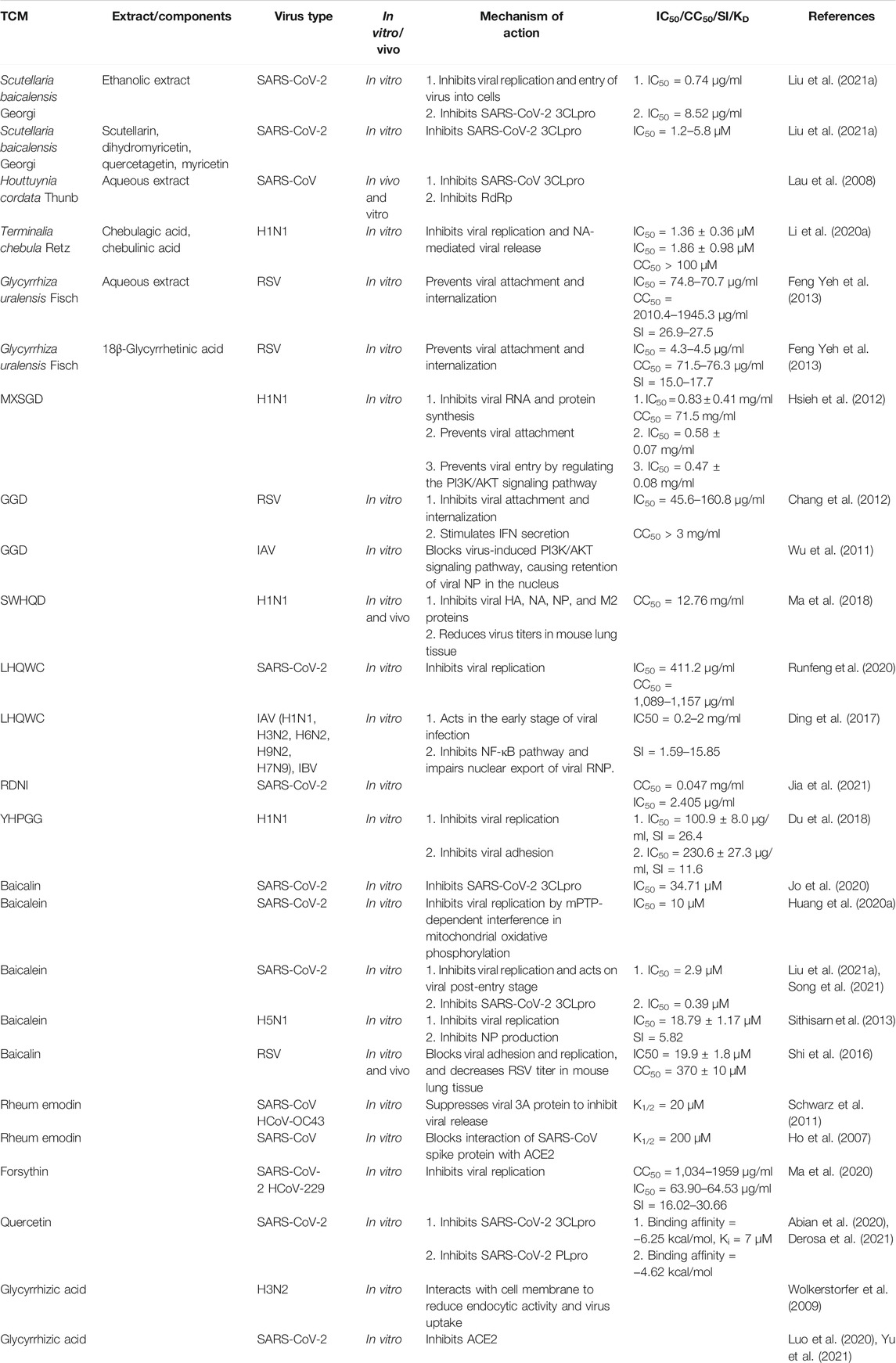
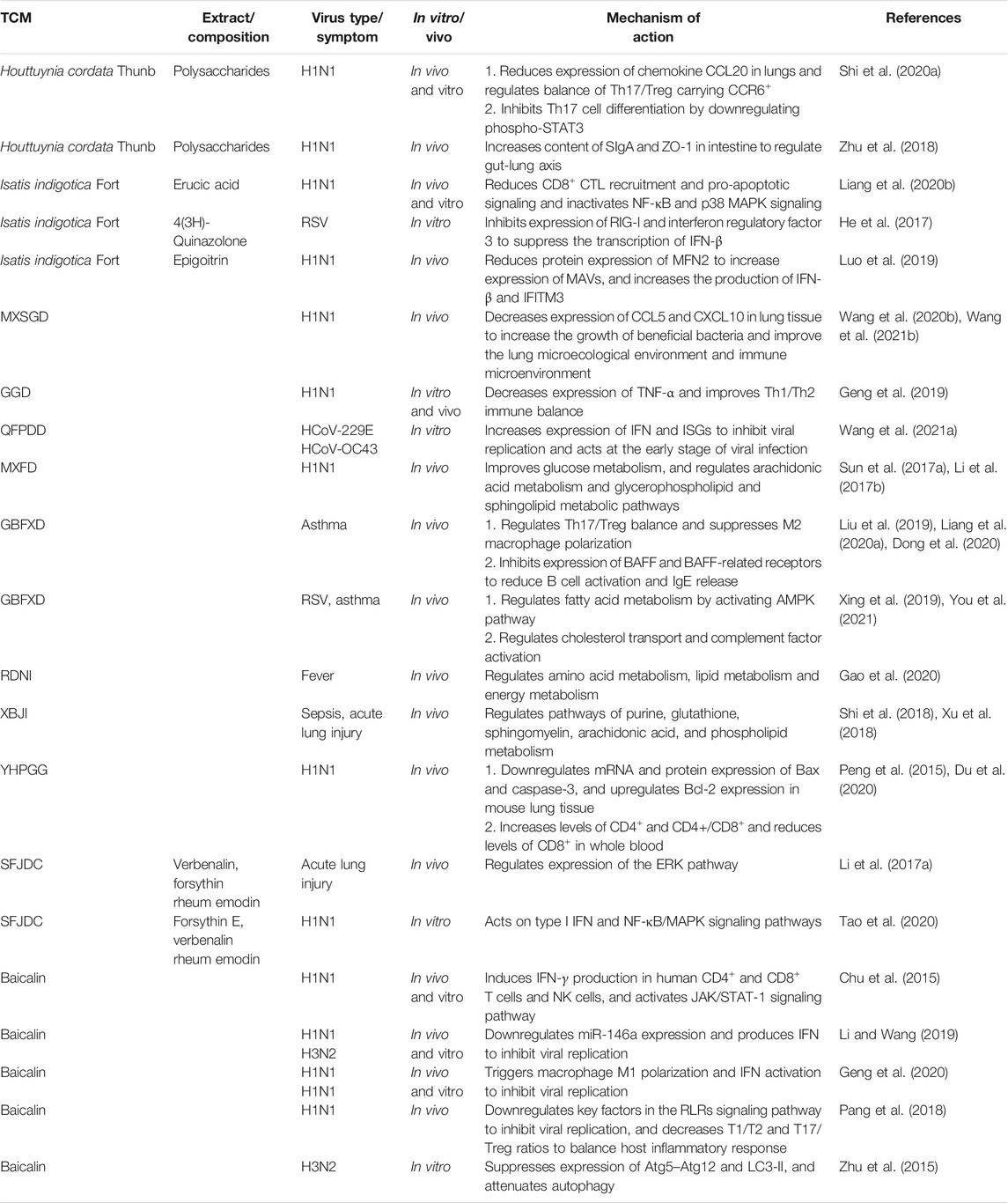
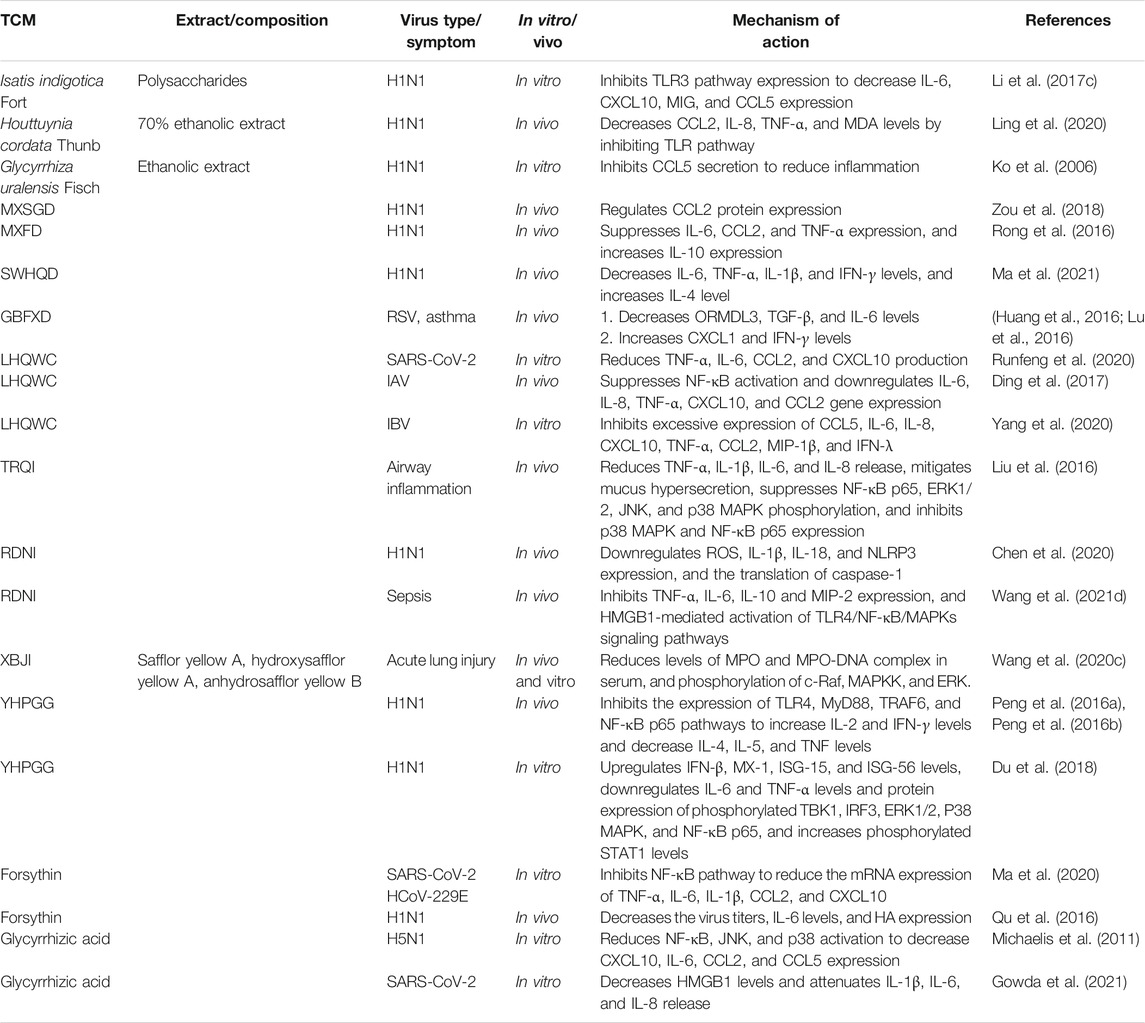
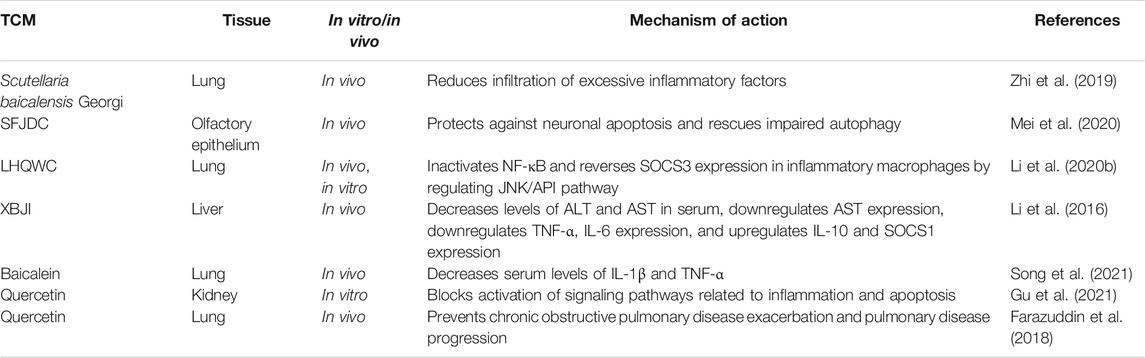
This review focuses on five Chinese herbal medicines (Isatidis radix, Glycyrrhizae radix et rhizoma, Scutellariae radix, Houttuyniae herba, and Chebulae fructus), six traditional Chinese formulae (Ge-gen decoction [GGD], San-wu-huang-qin decoction [SWHQD], Gu-ben-fang-xiao decoction [GBFXD], Qing-fei-pai-du decoction [QFPDD], Ma-xing-shi-gan decoction [MXSGD], and Ma-huang-xi-xin-fu-zi decoction [MXFD]), six proprietary Chinese medicines (Lian-hua-qing-wen capsules [LHQWC], Yin-hua-ping-gan granules [YHPGG], Shu-feng-jie-du capsules [SFJDC], Re-du-ning injection [RDNI], Xue-bi-jing injection [XBJI], and Tan-re-qing injection [TRQI]), and six active ingredients from natural products that have been studied extensively (forsythin, rheum emodin, baicalein, baicalin, quercetin, and glycyrrhizic acid) (Table 1). Based on a modern biological interpretation of TCM antiviral theory, we discuss the efficacy and mechanism of TCMs against respiratory viruses from the perspectives of the direct effect on viruses (Table 2), immune regulation (Table 3), control of inflammatory factors (Table 4), and tissue protection (Table 5).
Direct-Acting Antivirals
Taking respiratory viruses, such as high-risk coronaviruses (SARS-CoV and SARS-CoV-2), IAV, and RSV, as examples, the direct inhibitory effects of TCMs on respiratory viruses include interference with viral adsorption and invasion, replication (e.g., transcription and translation, nuclear output, and assembly), packaging, and budding (Figure 2; Table 2).
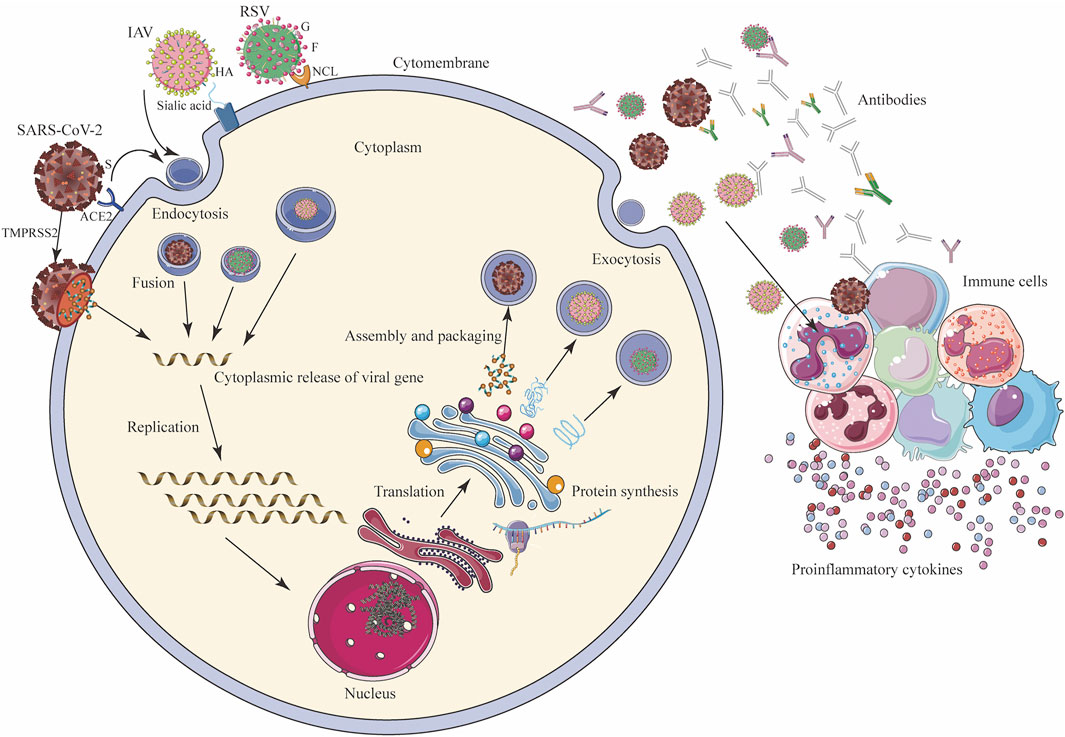
FIGURE 2. Mechanism of viral infection (SARS-CoV-2, IAV, and RSV) of host cells and host immune response. SARS-CoV-2 binds to ACE2, and IAV interacts with sialic acid through the HA on the surface and enters the host cells by endocytosis, and SARS-CoV-2 can directly enter cells under the action of TMPRSS2. G protein on the surface of RSV adheres to the cell membrane, and F protein binds to NCL and endocytosis into cells. After entering the cell, the virus releases its genome in the cytoplasm. Through transcription and translation in the nucleus, it is exported to the nuclear endoplasmic reticulum and ribosomes for the synthesis and assembly of viral proteins, and finally forms new progeny virus particles, which are exported to the outside of the cell in the form of exocytosis, and the host’s immune system While being activated, immune cells secrete a large number of antibodies and cytokines to fight the virus. ACE2: angiotensin converting enzyme 2; HA: hemagglutinin; G: glyco protein; F: fusion protein; NCL: nucleolin receptor; S: spike protein; TMPRSS2: transmembrane protease, serine 2.
Inhibition of Viral Adsorption and Invasion
The interaction between the viral surface protein and the host cell surface receptor is key for how the virus enters the cell; for example, the spike protein and angiotensin converting enzyme 2 (ACE2) receptor for SARS-CoV and SARS-CoV-2, hemagglutinin (HA) and the sialic acid protein for IAV, and the fusion protein and nucleolin receptor for RSV (Griffiths et al., 2020). Chinese herbal medicine aimed at the surface proteins or host receptors of these viruses can effectively “keep the enemy out of the country”. For example, RDNI acts on ACE2 to inhibit SARS-CoV-2 invasion, and effectively blocks viral replication in cells by inhibiting the main protease, resulting in a dual-target protective effect (Jia et al., 2021). The active component of LHQWC shared 189 common proteins with ACE2 co-expression proteins, which interact with each other (Zheng et al., 2020) and exert a multi-target synergistic effect that may prevent drug resistance caused by using a single ACE2 inhibitor (Runfeng et al., 2020; Yang et al., 2020; Chen et al., 2021a). LHQWC was the first drug approved to treat COVID-19 in China during the pandemic due to its clinical efficacy. Further studies showed that rheum emodin blocks the binding of the SARS-CoV spike protein to ACE2 and inhibits virus infection with a K1/2 value of about 200 μM(Ho et al., 2007). In addition, glycyrrhizic acid acts on the ACE2 receptor and prevents SARS-CoV-2 from entering cells (Luo et al., 2020; Yu et al., 2021).
In influenza viruses, MXSGD targets HA protein and regulates the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathway to block viral entry, blocks H1N1 virus RNA replication and protein synthesis, and has a synergistic effect with oseltamivir (Hsieh et al., 2012). Glycyrrhizic acid acts on the cell membrane, reduces its endocytosis activity, inhibits the entry of IAV into cells, and thus reduces virus uptake (Wolkerstorfer et al., 2009).
In RSV invasion, GGD may inhibit RSV fusion protein, inhibit viral adsorption and invasion, and stimulate host mucosal cells to produce interferon (IFN)-β (Chang et al., 2012). In vitro studies have shown that aqueous licorice extract and glycyrrhetinic acid can inhibit RSV attachment and entry into host cells (Feng Yeh et al., 2013). Baicalin can block pre-infection by directly killing RSV(Shi et al., 2016).
Inhibition of Viral Replication
In SARS-CoV viral replication, 3C-Like protease (3CLpro), papain-like protease (PLpro), and RNA-dependent RNA polymerase (RdRp) are the key proteases (Hilgenfeld, 2014), and are promising drug targets (Anand et al., 2003). The aqueous extract of Houttuynia cordata Thunb. inhibits SARS-CoV 3CLpro and RdRp in vitro (Lau et al., 2008). The ethanolic extract of Scutellaria baicalensis Georgi inhibits viral replication by acting on 3CLpro (Liu et al., 2021a). Baicalein interferes with mitochondrial oxidative phosphorylation (Huang et al., 2020a) and inhibits SARS-CoV-2 3CLpro in a mitochondrial permeability transition pore (mPTP)-dependent manner in vitro (Liu et al., 2021a; Song et al., 2021). Baicalin and other active components in Scutellaria baicalensis Georgi, such as scutellarin, dihydromyricetin, quercetagetin, and myricetin, also selectively inhibit SARS-CoV-2 3CLpro (Jo et al., 2020; Liu et al., 2021a). In addition, forsythin inhibits the replication of coronaviruses, such as SARS-CoV-2, in vitro (Ma et al., 2020; Su et al., 2020). Molecular docking has shown that quercetin inhibits the 3CLpro and PLpro targets of SARS-CoV-2 (Abian et al., 2020; Derosa et al., 2021).
The IAV genome encodes 11 genes, including those for neuraminidase (NA), matrix protein 1 (M1), matrix protein 2 (M2), HA, and nucleoprotein (NP). Blocking the release, replication, and synthesis of proteins related to influenza virus ribonucleoprotein (RNP) is effective for anti-influenza therapy (Dou et al., 2018; Umeoguaju et al., 2021). SWHQD inhibits the HA, NA, NP, and M2 ion channels of the influenza H1N1 virus and blocks the proliferation and replication of virus particles (Ma et al., 2018). GGD inhibits the PI3K/AKT pathway induced by IAV, resulting in the retention of virus RNP in the nucleus, and thus interferes with viral replication (Wu et al., 2011). YHPGG has shown the best inhibitory effect on the replication stage of H1N1 influenza virus with a selectivity index (SI) of 26.4 (Du et al., 2018). LHQWC inhibits different strains of SARS-CoV-2, IAV, and IBV. In the early stage of virus infection, LHQWC inhibits the activity of nuclear factor (NF)-κB, weakening the nuclear output of virus RNP and progeny reproduction, and, combined with oseltamivir, improved symptoms in IBV-infected mice (Ding et al., 2017). Meta-analysis showed that LHQWC is superior to oseltamivir in improving the symptoms of IAV infection and is similar to oseltamivir in clearing the virus without serious adverse reactions (Zhao et al., 2014). In addition, baicalein inhibits the production of H5N1 influenza virus NP and inhibits viral replication (Sithisarn et al., 2013).
Inhibition of Virus Release
In coronaviruses, the 3A ion channel mediates the virus release (Schwarz et al., 2014). Rheum emodin inhibits the 3A ion channel in coronaviruses, such as SARS-CoV, and inhibits the release of progeny virions with a K1/2 value of about 20 μM (Schwarz et al., 2011).
In IAV, the NA, HA, and M2 proteins are exported to the plasma membrane and used with viral RNP to produce IAV virions. Under NA catalysis, the newly assembled viruses are then transmitted from the host cell (Battles and McLellan, 2019; Blockus et al., 2020; Umeoguaju et al., 2021). The release of influenza virions is closely related to NA protein. The active components of the aqueous extract of Terminalia chebula Retz., chebulagic acid and chebulinic acid, inhibit the activity of viral NA protein, break the binding of virions to sialic acids on infected cells, block the virus release, and have a strong inhibitory effect on oseltamivir-resistant influenza strains (Li et al., 2020a).
Compared with small molecular inhibitors, TCMs have the advantage of containing multiple components and can have a synergistic effect on multiple targets. These multi-component, multi-target antiviral effects of TCMs support the basic theory of TCM.
Indirect Immune Regulation Against Viral Diseases
Many TCMs control the development of viral diseases by regulating the balance of the immune system and maintaining the stability of the internal environment of the body, which is the embodiment of the TCM principle of holism (Table 3).
Regulation of IFN Secretion
IFN is a broad-spectrum antiviral glycoprotein, which acts as a trigger, regulator, and effector of the immune system to participate in many physiological responses in virus infection (Malmgaard, 2004), and it is the most important cytokine (Richard, 2021). QFPDD upregulates the expression of IFN and interferon-stimulated genes (ISGs), and acts on the early stage of SARS-CoV-2 virus infection (Wang et al., 2021a). Forsythin E, forsythin, verbenalin, and rheum emodin, which are the important components of SFJDC, improve the symptoms of mice infected with the H1N1 influenza virus by regulating type 1 IFN, the NF-κB/mitogen-activated protein kinase (MAPK) signaling pathway, and the extracellular signal-regulated kinase (ERK) pathway (Li et al., 2017a; Tao et al., 2020). Clinical studies have shown that SFJDC combined with umifenovir treatment improves the immunity of ordinary COVID-19 patients, inhibits pulmonary inflammation, and shortens the average antipyretic time (Chen et al., 2021b). Isatis tinctoria L. cooperatively regulates the expression of IFN-β by inhibiting the retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein (MDA) 5 signaling pathways (Xu et al., 2019). The active components, tryptamine B, 4(3H)-quinazolone, and epigoitrin, activate the RIG-I signaling pathway, reduce the expression of mitochondrial fusion protein 2 (MFN2), and increase the expression of mitochondrial antiviral signal (MAVs), and thus promote IFN-β secretion (He et al., 2017; Luo et al., 2019). Baicalin downregulates the expression of miR-146a, which promotes IFN secretion and inhibits infection with H1N1 and H3N2 viruses (Li and Wang, 2019).
Regulation of Nonspecific and Humoral Immunity
After viral infection, cells recruit and activate macrophages, natural killer (NK) cells, and other immune cells by releasing cytokines, chemokines, and other signals to kill and eliminate infected cells. Once the regulation is out of balance, it causes an excessive immune response and tissue damage (Dai et al., 2020). GGD reduces the expression of the toll-like receptor (TLR) seven pathway signal and tumor necrosis factor (TNF)-α in H1N1 infected mice and improves the immune balance of T helper 1 (Th1) and T-helper 2 (Th2) cells (Geng et al., 2019). GBFXD inhibits the expression of B cell activating factor (BAFF) secreted by pulmonary macrophages and its related receptors, reduces the activation of B cells and the release of immunoglobulin E (IgE) (Liang et al., 2020a), inhibits the polarization activation of M2 macrophages, improves Th1/Th2 balance, reduces airway hyperresponsiveness and mucus secretion (Liu et al., 2019), regulates cholesterol transport, activates complement factors, and improves respiratory function and virus-induced asthma (Xing et al., 2019). YHPGG increases the level of CD4+T cells and the ratio of CD4+/CD8+ cells in peripheral blood of mice infected with H1N1, decreases the level of CD8+T cells (Peng et al., 2015), downregulates B-cell lymphoma 2 (Bcl-2)-associated X (Bax) and caspase-3 expression in mouse lung tissue, upregulates the expression of Bcl-2, and regulates apoptosis induced by virus (Du et al., 2020). Erucic acid in Isatis tinctoria L. reduces recruitment of CD8+ cytotoxic T lymphocytes (CTL), inhibits pro-apoptotic signals and NF-κB/MAPK signals, and reduces pulmonary inflammation (Liang et al., 2020b). Baicalin inhibits H1N1 infection by directly inducing human CD4+, CD8+T, and NK cells to produce IFN-γ and activating the JAK/STAT-1 signaling pathway (Chu et al., 2015) and by inducing macrophage M1 polarization and IFN activation (Geng et al., 2020). Furthermore, baicalin regulates key factors in the RIG-I-like receptors (RLRs) signaling pathway, inhibits H1N1 influenza viral replication, reduces the Th1/Th2 and T helper 17 (Th17)/regulatory T cells (Treg) ratios, and limits immunopathological damage (Pang et al., 2018). Baicalin also regulates the mTOR signaling pathway to inhibit the expression of the autophagy elongation complex (ATG5-Atg12) and lipidated LC3 (LC3-II), and inhibits autophagy induced by H3N2 influenza virus (Zhu et al., 2015).
Regulation of Intestinal Immunity
The lung and intestine originate from the same germinal layer in embryology and participate in mucus immunity (Barfod et al., 2013; Segal and Blaser, 2014; Wang and Tian, 2015). According to the theory of TCM, the lung and large intestine have an exterior-interior relationship. Intestinal disorders may affect the immune balance of lung tissue (Lee et al., 2021). When the intestinal barrier is damaged, pathogenic bacteria are exposed and transferred by M cells in the lymphoid follicular epithelium (Swank and Deitch, 1996), and infection of dendritic cells (DC) in gut-associated lymphoid tissue (GALT) activates T cell subsets in the mesenteric lymph node to produce regulatory cytokines (Wang et al., 2014). Intestinal mucosal immunity, which is central to the lung-intestinal axis, affects both the lung and the intestine (Zhu et al., 2018). MXSGD reduces the relative abundance of bacteria in the lung and intestine by reducing the levels of chemokines CC chemokine ligand (CCL) 5 and CXC motif chemokine ligand (CXCL) 10 in the lung tissue, promotes the growth of beneficial bacteria in the lung, improves the lung immune microecological environment, and protects the lungs from injury caused by the H1N1 influenza virus(Wang et al., 2020b; Wang et al., 2021b). Polysaccharides from Houttuynia cordata Thunb. promote specific migration of Th17CCR6+/TregCCR6+ cells from GALT to the lungs and regulate the Th17/Treg balance in IAV-infected mice (Shi et al., 2020a). In addition, these polysaccharides increase the levels of intestinal secretory immunoglobulin A (SIgA) and zonula occludens 1 (ZO-1), improve the intestinal physical barrier and immune barrier, inhibit the expression of TLR4 and p-NF-κB p65 in lung tissue, and reduce mortality in H1N1-infected mice (Zhu et al., 2018).
Regulation of Metabolism
Metabonomics is an important applied research method for the TCM principle of holism and uses a top-down strategy to understand physiological changes by analyzing the function of the organism based on the final effects in the metabolic network detected with modern techniques. The metabolic analysis of serum and feces by high-performance liquid chromatography time-of-flight mass spectrometry (TOF-MS) revealed that MXFD may exert an antiviral effect via mechanisms including improving energy metabolism and regulating arachidonic acid metabolism, glycerol phospholipid metabolism, the tricarboxylic acid cycle, tryptophan metabolism, and vitamin B6 metabolism (Sun et al., 2017a; Li et al., 2017b). Serum metabonomics showed that GBFXD activates the AMP-activated protein kinase (AMPK) pathway to regulate fatty acid metabolism and lipid metabolism and maintain the dynamic balance of lipids on the lung surface, and thus reduce asthma symptoms (You et al., 2021). Ultra-performance liquid chromatography to quadrupole (UPLC-Q)-TOF-MS analysis showed that RDNI regulates amino acid metabolism, lipid metabolism, and energy metabolism in febrile rats (Gao et al., 2020). Metabonomic analysis based on UHPLC-Q-Orbitrap high-resolution MS showed that XBJI reduces lung injury caused by sepsis by regulating energy metabolism, amino acid metabolism, fat metabolism, fatty acid metabolism, and hormone metabolism (Xu et al., 2018).
From the perspective of the principle of holism, TCMs regulate immune balance in many ways, such as IFN level, specific and non-specific immunity, intestinal immunity, and metabolism, and they reduce excessive immune reactions, suppress viral infection, improve pathological injury, and recover normal physiological function and the homeostasis of the internal environment.
Control of Inflammatory Factors Induced by Viral Infection
Inflammation is part of the body’s immune system that helps to control viruses, but it can also cause pathological damage. Uncontrolled inflammation can trigger a cytokine storm, leading to cytokine release syndrome (Zuo et al., 2020), tissue damage to the heart, liver, and kidneys, respiratory and multiple organ failure, and even death (Schett et al., 2020; Huang et al., 2020b). Therefore, anti-inflammatory drugs are as important as antiviral drugs for critical patients (Table 4)(Ren et al., 2020; Zhang et al., 2020; Chikhale et al., 2021; Wang et al., 2021c).
Control of Inflammatory Factors to Prevent a Cytokine Storm
TCMs can synergistically regulate the release of cytokines and chemokines via multiple targets and pathways. MXFD inhibits the expression of interleukin (IL)-6, CCL2, and TNF-α in serum, increases the expression of IL-10 and reduces inflammatory reaction (Rong et al., 2016). SWHQD reduces the levels of IL-6, TNF-α, IL-1β, and IFN-γ, and increases the level of IL-4 in serum, bronchoalveolar lavage fluid (BALF), and lung tissue of H1N1 infected mice (Ma et al., 2021). MXSGD downregulates the expression of CCL2 protein in lung tissue (Zou et al., 2018). YHPGG increases IL-2 and TNF-γ levels and decreases IL-4, IL-5, and TNF levels in H1N1-infected mice by inhibiting the expression of the TLR4/myeloid differentiation primary response protein 88 (MyD88)/TNF receptor-associated factor 6 (TRAF6) signaling pathway and NF-κB p65 (Peng et al., 2016a; Peng et al., 2016b). In addition, YHPGG upregulates the levels of TNF-β and ISGs, such as Mx-1, isg-15, and isg-56, and regulates the protein expression of key effectors in the type I IFN and pattern recognition receptor signaling pathway (Du et al., 2018). LHQWC inhibits the cytokines TNF-α, IL-6, CCL2, and CXCL10 induced by SARS-CoV-2 in vitro (Runfeng et al., 2020); inhibits the activation of NF-κB induced by IAV and IBV; inhibits the gene expression of IL-6, IL-8, TNF-α, CXCL10, CCL2, and TNF-λ; and prevents severe inflammation (Ding et al., 2017; Yang et al., 2020). A randomized, double-blind, controlled clinical trial showed that the average antipyretic time of RDNI in the treatment of seasonal influenza was no longer than that of oseltamivir, and there were no serious adverse reactions (Liu et al., 2017). Treatment with ribavirin decreases the expression of reactive oxygen species (ROS) in lung tissue, downregulates IL-1β and IL-18 levels, and inhibits the activation of NLR family pyrin domain containing 3 (NLRP3) inflammatory bodies (Chen et al., 2020). Polysaccharides of Isatis tinctoria L. inhibit the expression of TLR3, and thus inhibit the secretion of CXCL10, IL-6, MIG, and CCL5(Li et al., 2017c). The 70% ethanolic extract of Houttuynia cordata Thunb. decreases the phosphorylation and nuclear translocation of TLR3/4/7 and NF-κB p65, and decreases the levels of CCL2, IL-8, TNF-α, and MDA (Ling et al., 2020). Glycyrrhizic acid reduces the activation of NF-κB, c-Jun N-terminal kinases (JNKs), and p38 and inhibits the expression of pro-inflammatory molecules, such as CXCL10, IL-6, CCL2, and CCL5, by inhibiting ROS formation induced by H5N1 (Michaelis et al., 2011). Forsythin reduces the production of proinflammatory cytokines TNF-α, IL-6, IL-1β, CCL2, and CXCL10 and alleviates cytokine storm caused by SARS-CoV-2 and human coronavirus (HCoV)-229E by regulating the NF-κB signaling pathway (Ma et al., 2020). In H1N1-infected mice, forsythin reduces the level of IL-6 and lung tissue injury (Qu et al., 2016).
Relief of Respiratory Symptoms Caused by Inflammation
Respiratory viruses cause acute asthma and airway inflammation via several mechanisms (Shi et al., 2020b). However, GBFXD increases the levels of CXCL1 and IFN-γ in the lungs and reduces the airway inflammation caused by RSV-ovalbumin (Lu et al., 2016). GBFXD may also prevent chronic asthma by reducing the levels of transforming growth factor (TGF)-β and IL-6, reducing the deposition of collagen in the airway, inhibiting the production of airway mucus, and downregulating the expression of orosomucoid like 3 (ORMDL3) (Huang et al., 2016). TRQI reduces the release of TNF-α, IL-1β, IL-6, and IL-8 in mouse lung tissue, reduces the entry of cytokines into BALF, reduces mucus secretion, regulates the NF-κB/MAPK signaling pathway, and alleviates respiratory tract inflammation (Liu et al., 2016). CCL5 plays an important role in activating and recruiting leukocytes to the inflammatory site. The ethanolic extract of Glycyrrhiza uralensis Fish.; Glycyrrhiza inflata Bat.; Glycyrrhiza glabra L. significantly inhibits the secretion of CCL5 by human bronchial epithelial cells induced by the H1N1 influenza virus (Ko et al., 2006).
Relief of Severe Inflammation
Severe COVID-19 patients exhibit symptoms including dyspnea, acute respiratory distress syndrome, and sepsis. At this stage, the mortality rate of patients is about 15% (Godeau et al., 2021). XBJI is the only proprietary Chinese medicine approved in China for treating sepsis, and it significantly shortens the improvement time for major clinical symptoms and hospital stays (Luo et al., 2021). The anti-inflammatory effect of XBJI arises from the regulation of the NF-κB signaling pathway (Zhou et al., 2021). Safflor yellow A, hydroxysafflor yellow A, and anhydrosafflor yellow B, the three main components of XBJI, inhibit increases in the levels of inflammatory factors in mouse BALF, reduce the level of plasma myeloperoxidase (MPO)-DNA complex, and decrease the phosphorylation of RAF proto-oncogene serine/threonine-protein kinase (c-RAF), mitogen-activated protein kinase kinase (MAPKK), and ERK in mouse lung tissue (Wang et al., 2020c). High mobility group protein B1 (HMGB1) is an important late inflammatory factor and an endogenous danger signal in the pathological process of sepsis (Andersson and Tracey, 2003). Luteolin, the active component of RDNI, inhibits the activation of the TLR4/NF-κB/MAPK signaling pathway mediated by HMGB1 (Wang et al., 2021d). Glycyrrhizic acid also inhibits the increase in HMGB1 after SARS-CoV-2 infection, reduces the levels of proinflammatory cytokines IL-1 β, IL-6, and IL-8, and alleviates severe inflammation (Gowda et al., 2021).
The infiltration of inflammatory factors induced by viruses can cause a variety of pathological damage in the focus tissue. TCMs can not only control cytokines and chemokines in many ways to prevent a cytokine storm, but can also protect critical patients, which demonstrates that TCMs can maintain the homeostasis of the internal environment of the body owing to their multiple components and targets.
Tissue Protection
Respiratory viruses first invade the patient’s lungs, causing varying degrees of lung injury, and the viral infection may become systemic (Synowiec et al., 2021). Therapeutic drugs may also increase the load on the liver, kidneys, and other tissues, resulting in multi-tissue injury. TCMs can protect lung tissue and improve multiple organ function by inhibiting excessive apoptosis, inflammation, and immune reaction (Table 5).
LHQWC regulates the JNK/activator protein one signaling pathway, reduces the activity of NF-κB in macrophages, reverses the expression of suppressor of cytokine signaling (SOCS) three and the abnormal expression of TNF-related apoptosis-inducing ligand, protects cells from apoptosis, and alleviates acute lung injury in mice (Li et al., 2020b). The ethanolic extract of Scutellaria baicalensis Georgi reduces IL-6, TNF-α, and CCL2 levels in the lung tissue of H1N1-infected mice, increases IL-10 and IFN-γ production, and protects lung tissue, which is superior to the effect of active component baicalein alone (Zhi et al., 2019). In addition, baicalein inhibits the infiltration of inflammatory cells in lung tissue after RSV infection, decreases the serum levels of IL-1β and TNF-α, and improves respiratory function in acute lung injury in mice (Song et al., 2021). Quercetin reduces the inflammatory reaction and pathological deterioration of lung tissue in mice with chronic obstructive pulmonary disease induced by rhinovirus (Farazuddin et al., 2018).
In addition to protecting lung tissue, XBJI reduces the levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), downregulates the expression of TNF-α and IL-6, upregulates the expression of IL-10 and SOCS1, and reduces liver injury caused by inflammation in rats (Li et al., 2016). Quercetin regulates apoptosis-related signaling pathways, blocks the inflammatory response, and protects against SARS-COV-2-induced acute kidney injury (Gu et al., 2021). Dysosmia is a common symptom in COVID-19 patients (Tong et al., 2020). SFJDC reduces the levels of IgE, TNF-α, and IL-1β in peripheral blood, lung tissue, and olfactory epithelial (OE) tissue of rats, prevents nerve cell apoptosis, rescues autophagy of damaged cells in lung and OE tissue, and protects OE neurons and lung tissue (Mei et al., 2020).
TCM regards the body as an organic whole, and thus not only regulates immune and inflammatory responses, but also prevents and protects from various pathological tissue injuries, resulting in mutual balance among various physiological functions. This overall stability and harmony are fundamental to disease prevention and health maintenance.
Development Prospects of Antiviral TCMs
TCMs have become a main focus of antiviral research because of their advantages, including reliable clinical efficacy, few side effects, and low drug resistance, which arise from the principles of holism and syndrome differentiation and treatment. Therefore, to study the antiviral properties of TCMs and develop new TCMs, we must combine the basic theory of TCM with the latest research in Western medicine and modern biological science. In emergencies, Western medicine emphasizes using compounds that target a single receptor to relieve symptoms quickly at the disease site, which is effective, but not always sufficient to restore the functional balance of the body, making adverse reactions particularly obvious. For example, during the SARS epidemic in 2003, high-dose glucocorticoid treatment caused serious side effects in critical patients, including immunosuppression, delayed virus clearance, and bone destruction (Choudhry et al., 2020). Holistic treatment with TCMs, which have multiple components and targets, focuses on the interactions and relationships among the body, viruses, and drugs, and has considerable advantages in adaptability and effectiveness in the treatment of complex human diseases that cause immune imbalance, especially during an outbreak of an unknown new virus. This holistic philosophy is also being used in the emerging field of network pharmacology, and is recognized in modern research methods, such as network biology and metabonomics.
The greatest advantage of TCM is the coordination of multiple components and targets. Because of the many components, TCM does not rely on a single antiviral mechanism of action, but harnesses the coexistence and interaction of multiple mechanisms. However, there is still insufficient research on the chemical component analysis and mechanism of action of TCMs, which restricts the development of TCM, and is also an obvious short board in the modern medical system. (Li et al., 2018). And research on the safety of TCMs in the treatment of viral diseases are not sufficient. “Safe” of TCMs is not the same as “natural”. Some TCMs have endogenous toxicity to organs such as liver and kidney, and also exogenous toxicity in the process of cultivation, processing, storage and distribution (Li et al., 2020c). Therefore, the further development of TCMs in the world must determine its side effects. Under the guidance of TCM theory, research on the mechanism of TCMs should examine the relationship of infection and immune response between virus and host directly, revealing the dynamic relationships between viral load, cytokines, and immune response. This information may reveal new insights that are difficult to discover via traditional biology, explain the mechanism of prevention and treatment of viruses with herbs using the technology and language of modern life sciences, and promote the deep integration of TCM and modern biotechnology.
Author Contributions
Conceptualization, Q-HC and B-HL; writing—original draft preparation, B-HL and Q-HC; writing—review and editing, B-HL, Z-YL, M-ML and Q-HC; funding acquisition, J-ZT and Q-HC. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shandong Provincial Natural Science Foundation, China, (Grant No. ZR2020MH383) and Key Research and Development Program of Shandong Province, China, (Major Science and Technology Innovation Project) (Grant No. 2020CXGC010505).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelrahman, Z., Li, M., and Wang, X. (2020). Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 11, 552909. doi:10.3389/fimmu.2020.552909
Abian, O., Ortega-Alarcon, D., Jimenez-Alesanco, A., Ceballos-Laita, L., Vega, S., Reyburn, H. T., et al. (2020). Structural Stability of SARS-CoV-2 3CLpro and Identification of Quercetin as an Inhibitor by Experimental Screening. Int. J. Biol. Macromol 164, 1693–1703. doi:10.1016/j.ijbiomac.2020.07.235
Anand, K., Ziebuhr, J., Wadhwani, P., Mesters, J. R., and Hilgenfeld, R. (2003). Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs. Science 300 (5626), 1763–1767. doi:10.1126/science.1085658
Andersson, U., and Tracey, K. J. (2003). HMGB1 in Sepsis. Scand. J. Infect. Dis. 35 (9), 577–584. doi:10.1080/00365540310016286
Barfod, K. K., Roggenbuck, M., Hansen, L. H., Schjørring, S., Larsen, S. T., Sørensen, S. J., et al. (2013). The Murine Lung Microbiome in Relation to the Intestinal and Vaginal Bacterial Communities. BMC Microbiol. 13, 303. doi:10.1186/1471-2180-13-303
Battles, M. B., and McLellan, J. S. (2019). Respiratory Syncytial Virus Entry and How to Block it. Nat. Rev. Microbiol. 17 (4), 233–245. doi:10.1038/s41579-019-0149-x
Blockus, S., Sake, S. M., Wetzke, M., Grethe, C., Graalmann, T., Pils, M., et al. (2020). Labyrinthopeptins as Virolytic Inhibitors of Respiratory Syncytial Virus Cell Entry. Antivir. Res 177, 104774. doi:10.1016/j.antiviral.2020.104774
Burney, P., Jarvis, D., and Perez-Padilla, R. (2015). The Global burden of Chronic Respiratory Disease in Adults. Int. J. Tuberc. Lung Dis. 19 (1), 10–20. doi:10.5588/ijtld.14.0446
Chang, J. S., Wang, K. C., Shieh, D. E., Hsu, F. F., and Chiang, L. C. (2012). Ge-Gen-Tang Has Anti-viral Activity against Human Respiratory Syncytial Virus in Human Respiratory Tract Cell Lines. J. Ethnopharmacol 139 (1), 305–310. doi:10.1016/j.jep.2011.11.018
Chen, J., Lin, S., Niu, C., and Xiao, Q. (2021). Clinical Evaluation of Shufeng Jiedu Capsules Combined with Umifenovir (Arbidol) in the Treatment of Common-type COVID-19: a Retrospective Study. Expert Rev. Respir. Med. 15 (2), 257–265. doi:10.1080/17476348.2020.1822741
Chen, W., Liang, W., Li, S., Wu, L., Cui, Y., Qi, Q., et al. (2017). Quality Evaluation of Terminalia billerica Based on HPLC Fingerprint and Multi-Components Simultaneous Determination. Chin. Traditional Herbal Drugs 48 (06), 1210–1215.
Chen, W., Ma, Y., Zhang, H., Guo, Y., Guan, M., and Wang, Y. (2020). Reduning Plus Ribavirin Display Synergistic Activity against Severe Pneumonia Induced by H1N1 Influenza A Virus in Mice. J. Tradit Chin. Med. 40 (5), 803–811. doi:10.19852/j.cnki.jtcm.2020.05.010
Chen, X., Wu, Y., Chen, C., Gu, Y., Zhu, C., Wang, S., et al. (2021). Identifying Potential Anti-COVID-19 Pharmacological Components of Traditional Chinese Medicine Lianhuaqingwen Capsule Based on Human Exposure and ACE2 Biochromatography Screening. Acta Pharm. Sin B 11 (1), 222–236. doi:10.1016/j.apsb.2020.10.002
Chikhale, R., Sinha, S. K., Wanjari, M., Gurav, N. S., Ayyanar, M., Prasad, S., et al. (2021). Computational Assessment of Saikosaponins as Adjuvant Treatment for COVID-19: Molecular Docking, Dynamics, and Network Pharmacology Analysis. Mol. Divers. 25, 1889–1904. doi:10.1007/s11030-021-10183-w
Choudhry, N., Zhao, X., Xu, D., Zanin, M., Chen, W., Yang, Z., et al. (2020). Chinese Therapeutic Strategy for Fighting COVID-19 and Potential Small-Molecule Inhibitors against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Med. Chem. 63 (22), 13205–13227. doi:10.1021/acs.jmedchem.0c00626
Chu, M., Xu, L., Zhang, M. B., Chu, Z. Y., and Wang, Y. D. (2015). Role of Baicalin in Anti-influenza Virus A as a Potent Inducer of IFN-Gamma. Biomed. Res. Int. 2015, 263630. doi:10.1155/2015/263630
Dai, Y. J., Wan, S. Y., Gong, S. S., Liu, J. C., Li, F., and Kou, J. P. (2020). Recent Advances of Traditional Chinese Medicine on the Prevention and Treatment of COVID-19. Chin. J. Nat. Med. 18 (12), 881–889. doi:10.1016/s1875-5364(20)60031-0
Derosa, G., Maffioli, P., D'Angelo, A., and Di Pierro, F. (2021). A Role for Quercetin in Coronavirus Disease 2019 (COVID-19). Phytother Res. 35 (3), 1230–1236. doi:10.1002/ptr.6887
Ding, Y., Zeng, L., Li, R., Chen, Q., Zhou, B., Chen, Q., et al. (2017). The Chinese Prescription Lianhuaqingwen Capsule Exerts Anti-influenza Activity through the Inhibition of Viral Propagation and Impacts Immune Function. BMC Complement. Altern. Med. 17 (1), 130. doi:10.1186/s12906-017-1585-7
Dong, Y., Yan, H., Zhao, X., Lin, R., Lin, L., Ding, Y., et al. (2020). Gu-Ben-Fang-Xiao Decoction Ameliorated Murine Asthma in Remission Stage by Modulating Microbiota-Acetate-Tregs Axis. Front. Pharmacol. 11, 549. doi:10.3389/fphar.2020.00549
Dou, D., Revol, R., Östbye, H., Wang, H., and Daniels, R. (2018). Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 9, 1581. doi:10.3389/fimmu.2018.01581
Du, H. X., Zhou, H. F., Wan, H. F., Yang, J. H., Lu, Y. Y., He, Y., et al. (2018). Antiviral Effects and Mechanisms of Yinhuapinggan Granule against H1N1 Influenza Virus Infection in RAW264.7 Cells. Inflammopharmacology 26 (6), 1455–1467. doi:10.1007/s10787-018-0457-1
Du, H. X., Zhou, H. F., Yang, J. H., Lu, Y. Y., He, Y., and Wan, H. T. (2020). Preliminary Study of Yinhuapinggan Granule against H1N1 Influenza Virus Infection in Mice through Inhibition of Apoptosis. Pharm. Biol. 58 (1), 979–991. doi:10.1080/13880209.2020.1818792
Farazuddin, M., Mishra, R., Jing, Y., Srivastava, V., Comstock, A. T., and Sajjan, U. S. (2018). Quercetin Prevents Rhinovirus-Induced Progression of Lung Disease in Mice with COPD Phenotype. PLoS One 13 (7), e0199612. doi:10.1371/journal.pone.0199612
Feng Yeh, C., Wang, K. C., Chiang, L. C., Shieh, D. E., Yen, M. H., and San Chang, J. (2013). Water Extract of Licorice Had Anti-viral Activity against Human Respiratory Syncytial Virus in Human Respiratory Tract Cell Lines. J. Ethnopharmacol 148 (2), 466–473. doi:10.1016/j.jep.2013.04.040
Gao, X., Huang, C., Geng, T., Chen, X., Wang, J., Liu, J., et al. (2020). Serum and Urine Metabolomics Based on UPLC-Q-TOF/MS Reveals the Antipyretic Mechanism of Reduning Injection in a Rat Model. J. Ethnopharmacol 250, 112429. doi:10.1016/j.jep.2019.112429
Garten, R. J., Davis, C. T., Russell, C. A., Shu, B., Lindstrom, S., Balish, A., et al. (2009). Antigenic and Genetic Characteristics of Swine-Origin 2009 A(H1N1) Influenza Viruses Circulating in Humans. Science 325 (5937), 197–201. doi:10.1126/science.1176225
Geng, P., Zhu, H., Zhou, W., Su, C., Chen, M., Huang, C., et al. (2020). Baicalin Inhibits Influenza A Virus Infection via Promotion of M1 Macrophage Polarization. Front. Pharmacol. 11, 01298. doi:10.3389/fphar.2020.01298
Geng, Z. K., Li, Y. Q., Cui, Q. H., Du, R. K., and Tian, J. Z. (2019). Exploration of the Mechanisms of Ge Gen Decoction against Influenza A Virus Infection. Chin. J. Nat. Med. 17 (9), 650–662. doi:10.1016/s1875-5364(19)30079-2
Godeau, D., Petit, A., Richard, I., Roquelaure, Y., and Descatha, A. (2021). Return-to-work, Disabilities and Occupational Health in the Age of COVID-19 Scand. J. Work Environ. Health (Helsinki, Finland: Scand J Work Environ Health), 47, 408–409. doi:10.5271/sjweh.3960
Gowda, P., Patrick, S., Joshi, S. D., Kumawat, R. K., and Sen, E. (2021). Glycyrrhizin Prevents SARS-CoV-2 S1 and Orf3a Induced High Mobility Group Box 1 (HMGB1) Release and Inhibits Viral Replication. Cytokine 142, 155496. doi:10.1016/j.cyto.2021.155496
Griffiths, C. D., Bilawchuk, L. M., McDonough, J. E., Jamieson, K. C., Elawar, F., Cen, Y., et al. (2020). IGF1R Is an Entry Receptor for Respiratory Syncytial Virus. Nature 583 (7817), 615–619. doi:10.1038/s41586-020-2369-7
Gu, Y. Y., Zhang, M., Cen, H., Wu, Y. F., Lu, Z., Lu, F., et al. (2021). Quercetin as a Potential Treatment for COVID-19-Induced Acute Kidney Injury: Based on Network Pharmacology and Molecular Docking Study. PLoS One 16 (1), e0245209. doi:10.1371/journal.pone.0245209
He, L., Fan, F., Hou, X., Wu, H., Wang, J., Xu, H., et al. (2017). 4(3H)-Quinazolone Regulates Innate Immune Signaling upon Respiratory Syncytial Virus Infection by Moderately Inhibiting the RIG-1 Pathway in RAW264.7 Cell. Int. Immunopharmacol 52, 245–252. doi:10.1016/j.intimp.2017.09.010
Hilgenfeld, R. (2014). From SARS to MERS: Crystallographic Studies on Coronaviral Proteases Enable Antiviral Drug Design. Febs j 281 (18), 4085–4096. doi:10.1111/febs.12936
Ho, T. Y., Wu, S. L., Chen, J. C., Li, C. C., and Hsiang, C. Y. (2007). Emodin Blocks the SARS Coronavirus Spike Protein and Angiotensin-Converting Enzyme 2 Interaction. Antivir. Res 74 (2), 92–101. doi:10.1016/j.antiviral.2006.04.014
Hsieh, C. F., Lo, C. W., Liu, C. H., Lin, S., Yen, H. R., Lin, T. Y., et al. (2012). Mechanism by Which ma-xing-shi-gan-tang Inhibits the Entry of Influenza Virus. J. Ethnopharmacol 143 (1), 57–67. doi:10.1016/j.jep.2012.05.061
Huang, F., Li, Y., Leung, E. L., Liu, X., Liu, K., Wang, Q., et al. (2020). A Review of Therapeutic Agents and Chinese Herbal Medicines against SARS-COV-2 (COVID-19). Pharmacol. Res. 158, 104929. doi:10.1016/j.phrs.2020.104929
Huang, S., Liu, Y., Zhang, Y., Zhang, R., Zhu, C., Fan, L., et al. (2020). Baicalein Inhibits SARS-CoV-2/VSV Replication with Interfering Mitochondrial Oxidative Phosphorylation in a mPTP Dependent Manner. Signal. Transduct Target. Ther. 5 (1), 266. doi:10.1038/s41392-020-00353-x
Huang, Z., Gao, L., Zhao, X., Ling, H., and Chen, W. (2016). Effect of Gubenfangxiao Decoction on Respiratory Syncytial Virus-Induced Asthma and Expression of Asthma Susceptibility Gene Orosomucoid 1-like Protein 3 in Mice. J. Tradit Chin. Med. 36 (1), 101–106. doi:10.1016/s0254-6272(16)30015-2
Ji, S., Li, R., Wang, Q., Miao, W. J., Li, Z. W., Si, L. L., et al. (2015). Anti-H1N1 Virus, Cytotoxic and Nrf2 Activation Activities of Chemical Constituents from Scutellaria Baicalensis. J. Ethnopharmacol 176, 475–484. doi:10.1016/j.jep.2015.11.018
Jia, S., Luo, H., Liu, X., Fan, X., Huang, Z., Lu, S., et al. (2021). Dissecting the Novel Mechanism of Reduning Injection in Treating Coronavirus Disease 2019 (COVID-19) Based on Network Pharmacology and Experimental Verification. J. Ethnopharmacol 273, 113871. doi:10.1016/j.jep.2021.113871
Jia, W., Wang, C., Wang, Y., Pan, G., Jiang, M., Li, Z., et al. (2015). Qualitative and Quantitative Analysis of the Major Constituents in Chinese Medical Preparation Lianhua-Qingwen Capsule by UPLC-DAD-QTOF-MS. ScientificWorldJournal 2015, 731765. doi:10.1155/2015/731765
Jo, S., Kim, S., Kim, D. Y., Kim, M. S., and Shin, D. H. (2020). Flavonoids with Inhibitory Activity against SARS-CoV-2 3CLpro. J. Enzyme Inhib. Med. Chem. 35 (1), 1539–1544. doi:10.1080/14756366.2020.1801672
Ko, H. C., Wei, B. L., and Chiou, W. F. (2006). The Effect of Medicinal Plants Used in Chinese Folk Medicine on RANTES Secretion by Virus-Infected Human Epithelial Cells. J. Ethnopharmacol 107 (2), 205–210. doi:10.1016/j.jep.2006.03.004
Lau, K. M., Lee, K. M., Koon, C. M., Cheung, C. S., Lau, C. P., Ho, H. M., et al. (2008). Immunomodulatory and Anti-SARS Activities of Houttuynia Cordata. J. Ethnopharmacol 118 (1), 79–85. doi:10.1016/j.jep.2008.03.018
Lee, D. Y. W., Li, Q. Y., Liu, J., and Efferth, T. (2021). Traditional Chinese Herbal Medicine at the Forefront Battle against COVID-19: Clinical Experience and Scientific Basis. Phytomedicine 80, 153337. doi:10.1016/j.phymed.2020.153337
Li, A., Li, J., Bao, Y., Yuan, D., and Huang, Z. (2016). Xuebijing Injection Alleviates Cytokine-Induced Inflammatory Liver Injury in CLP-Induced Septic Rats through Induction of Suppressor of Cytokine Signaling 1. Exp. Ther. Med. 12 (3), 1531–1536. doi:10.3892/etm.2016.3476
Li, C., Sun, Q., Fu, Y., Gong, L., Yang, Y., Jiang, H., et al. (2017). Mechanism Analysis of Mice Model with H1N1 Influenza Virus Intervened by Mahuang Xixin Fuzi Tang Based on Fecal Metabolomics. Chin. J. Exp. Traditional Med. Formulae 23 (09), 74–79.
Li, C., Zang, C., Nie, Q., Yang, B., Zhang, B., and Duan, S. (2019). Simultaneous Determination of Seven Flavonoids, Two Phenolic Acids and Two Cholesterines in Tanreqing Injection by UHPLC-MS/MS. J. Pharm. Biomed. Anal. 163, 105–112. doi:10.1016/j.jpba.2018.08.058
Li, P., Cui, Q., Wang, L., Zhao, X., Zhang, Y., Manicassamy, B., et al. (2018). A Simple and Robust Approach for Evaluation of Antivirals Using a Recombinant Influenza Virus Expressing Gaussia Luciferase. Viruses 10 (6). doi:10.3390/v10060325
Li, P., Du, R., Wang, Y., Hou, X., Wang, L., Zhao, X., et al. (2020). Identification of Chebulinic Acid and Chebulagic Acid as Novel Influenza Viral Neuraminidase Inhibitors. Front. Microbiol. 11, 182. doi:10.3389/fmicb.2020.00182
Li, Q., Ran, Q., Sun, L., Yin, J., Luo, T., Liu, L., et al. (2020). Lian Hua Qing Wen Capsules, a Potent Epithelial Protector in Acute Lung Injury Model, Block Proapoptotic Communication between Macrophages, and Alveolar Epithelial Cells. Front. Pharmacol. 11, 522729. doi:10.3389/fphar.2020.522729
Li, R., and Wang, L. (2019). Baicalin Inhibits Influenza Virus A Replication via Activation of type I IFN Signaling by Reducing miR-146a. Mol. Med. Rep. 20 (6), 5041–5049. doi:10.3892/mmr.2019.10743
Li, S., Liu, C., Guo, F., Taleb, S. J., Tong, M., and Shang, D. (2020). Traditional Chinese Medicine as Potential Therapy for COVID-19. Am. J. Chin. Med. 48 (6), 1263–1277. doi:10.1142/s0192415x20500627
Li, Y., Chang, N., Han, Y., Zhou, M., Gao, J., Hou, Y., et al. (2017). Anti-inflammatory Effects of Shufengjiedu Capsule for Upper Respiratory Infection via the ERK Pathway. Biomed. Pharmacother. 94, 758–766. doi:10.1016/j.biopha.2017.07.118
Li, Y., Chu, F., Li, P., Johnson, N., Li, T., Wang, Y., et al. (2021). Potential Effect of Maxing Shigan Decoction against Coronavirus Disease 2019 (COVID-19) Revealed by Network Pharmacology and Experimental Verification. J. Ethnopharmacol 271, 113854. doi:10.1016/j.jep.2021.113854
Li, Z., Li, L., Zhou, H., Zeng, L., Chen, T., Chen, Q., et al. (2017). Radix Isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro. Molecules 22 (1). doi:10.3390/molecules22010116
Li, Z., and Xu, C. (2011). The Fundamental Theory of Traditional Chinese Medicine and the Consideration in its Research Strategy. Front. Med. 5 (2), 208–211. doi:10.1007/s11684-011-0126-x
Liang, X., Huang, Y., Pan, X., Hao, Y., Chen, X., Jiang, H., et al. (2020). Erucic Acid from Isatis Indigotica Fort. Suppresses Influenza A Virus Replication and Inflammation In vitro and In vivo through Modulation of NF-Κb and P38 MAPK Pathway. J. Pharm. Anal. 10 (2), 130–146. doi:10.1016/j.jpha.2019.09.005
Liang, X., Liu, C. S., Xia, T., Tang, Q. F., and Tan, X. M. (2020). Identification of Active Compounds of Mahuang Fuzi Xixin Decoction and Their Mechanisms of Action by LC-MS/MS and Network Pharmacology. Evid. Based Complement. Alternat Med. 2020, 3812180. doi:10.1155/2020/3812180
Liang, Z. Q., Tu, P. C., Ji, J. J., Xing, Q. Q., and Zhao, X. (2020). Gu-Ben-Fang-Xiao Attenuates Allergic Airway Inflammation by Inhibiting BAFF-Mediated B Cell Activation. Biomed. Pharmacother. 132, 110801. doi:10.1016/j.biopha.2020.110801
Ling, L. J., Lu, Y., Zhang, Y. Y., Zhu, H. Y., Tu, P., Li, H., et al. (2020). Flavonoids from Houttuynia Cordata Attenuate H1N1-Induced Acute Lung Injury in Mice via Inhibition of Influenza Virus and Toll-like Receptor Signalling. Phytomedicine 67, 153150. doi:10.1016/j.phymed.2019.153150
Liu, H., Ye, F., Sun, Q., Liang, H., Li, C., Li, S., et al. (2021). Scutellaria Baicalensis Extract and Baicalein Inhibit Replication of SARS-CoV-2 and its 3C-like Protease In Vitro. J. Enzyme Inhib. Med. Chem. 36 (1), 497–503. doi:10.1080/14756366.2021.1873977
Liu, L. W., Xing, Q. Q., Zhao, X., Tan, M., Lu, Y., Dong, Y. M., et al. (2019). Proteomic Analysis Provides Insights into the Therapeutic Effect of GU-BEN-FANG-XIAO Decoction on a Persistent Asthmatic Mouse Model. Front. Pharmacol. 10, 441. doi:10.3389/fphar.2019.00441
Liu, W., Jiang, H. L., Cai, L. L., Yan, M., Dong, S. J., and Mao, B. (2016). Tanreqing Injection Attenuates Lipopolysaccharide-Induced Airway Inflammation through MAPK/NF-κB Signaling Pathways in Rats Model. Evid. Based Complement. Alternat Med. 2016, 5292346. doi:10.1155/2016/5292346
Liu, W., Huang, J., Zhang, F., Zhang, C.-C., Li, R.-S., Wang, Y.-L., et al. (2021). Comprehensive Profiling and Characterization of the Absorbed Components and Metabolites in Mice Serum and Tissues Following Oral Administration of Qing-Fei-Pai-Du Decoction by UHPLC-Q-Exactive-Orbitrap HRMS. Chin. J. Nat. Medicines 19 (4), 305–320. doi:10.1016/s1875-5364(21)60031-6
Liu, Y., Mu, W., Xiao, W., Wei, B. L., Wang, L., Liu, X. Q., et al. (2017). Efficacy and Safety of Re-du-ning Injection in the Treatment of Seasonal Influenza: Results from a Randomized, Double-Blinded, Multicenter, Oseltamivir-Controlled Trial. Oncotarget 8 (33), 55176–55186. doi:10.18632/oncotarget.19220
Lu, Y., Xu, J. Y., Zhang, X. H., and Zhao, X. (2016). Gu-Ben-Fang-Xiao Decoction Attenuates Sustained Airway Inflammation by Suppressing ER Stress Response in a Murine Asthma Remission Model of Respiratory Syncytial Virus Infection. J. Ethnopharmacol 192, 496–509. doi:10.1016/j.jep.2016.09.039
Luo, P., Liu, D., and Li, J. (2020). Pharmacological Perspective: Glycyrrhizin May Be an Efficacious Therapeutic Agent for COVID-19. Int. J. Antimicrob. Agents 55 (6), 105995. doi:10.1016/j.ijantimicag.2020.105995
Luo, Z., Chen, W., Xiang, M., Wang, H., Xiao, W., Xu, C., et al. (2021). The Preventive Effect of Xuebijing Injection against Cytokine Storm for Severe Patients with COVID-19: A Prospective Randomized Controlled Trial. Eur. J. Integr. Med. 42, 101305. doi:10.1016/j.eujim.2021.101305
Luo, Z., Liu, L. F., Wang, X. H., Li, W., Jie, C., Chen, H., et al. (2019). Epigoitrin, an Alkaloid from Isatis Indigotica, Reduces H1N1 Infection in Stress-Induced Susceptible Model In Vivo and In Vitro. Front. Pharmacol. 10, 78. doi:10.3389/fphar.2019.00078
Ma, Q., Li, R., Pan, W., Huang, W., Liu, B., Xie, Y., et al. (2020). Phillyrin (KD-1) Exerts Anti-viral and Anti-inflammatory Activities against Novel Coronavirus (SARS-CoV-2) and Human Coronavirus 229E (HCoV-229E) by Suppressing the Nuclear Factor Kappa B (NF-Κb) Signaling Pathway. Phytomedicine 78, 153296. doi:10.1016/j.phymed.2020.153296
Ma, Q., Yu, Q., Xing, X., Liu, S., Shi, C., and Luo, J. (2018). San Wu Huangqin Decoction, a Chinese Herbal Formula, Inhibits Influenza a/PR/8/34 (H1N1) Virus Infection In Vitro and In Vivo. Viruses 10 (3). doi:10.3390/v10030117Epub 2018/03/10
Ma, Q. H., Ren, M. Y., and Luo, J. B. (2021). San Wu Huangqin Decoction Regulates Inflammation and Immune Dysfunction Induced by Influenza Virus by Regulating the NF-Κb Signaling Pathway in H1N1-Infected Mice. J. Ethnopharmacol 264, 112800. doi:10.1016/j.jep.2020.112800
Ma, Y., Chen, M., Guo, Y., Liu, J., Chen, W., Guan, M., et al. (2019). Prevention and Treatment of Infectious Diseases by Traditional Chinese Medicine: a Commentary. Apmis 127 (5), 372–384. doi:10.1111/apm.12928
Malmgaard, L. (2004). Induction and Regulation of IFNs during Viral Infections. J. Interferon Cytokine Res. 24 (8), 439–454. doi:10.1089/1079990041689665
Mei, J., Kong, H., Zhao, Z., Chen, Z., Wang, Y., and Yang, J. (2020). Shufengjiedu Capsules Protect against Neuronal Loss in Olfactory Epithelium and Lung Injury by Enhancing Autophagy in Rats with Allergic Rhinitis. Biosci. Trends 13 (6), 530–538. doi:10.5582/bst.2019.01332
Michaelis, M., Geiler, J., Naczk, P., Sithisarn, P., Leutz, A., Doerr, H. W., et al. (2011). Glycyrrhizin Exerts Antioxidative Effects in H5N1 Influenza A Virus-Infected Cells and Inhibits Virus Replication and Pro-inflammatory Gene Expression. PLoS One 6 (5), e19705. doi:10.1371/journal.pone.0019705
Moriyama, M., Hugentobler, W. J., and Iwasaki, A. (2020). Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 7 (1), 83–101. doi:10.1146/annurev-virology-012420-022445
Nichols, W. G., Peck Campbell, A. J., and Boeckh, M. (2008). Respiratory Viruses Other Than Influenza Virus: Impact and Therapeutic Advances. Clin. Microbiol. Rev. 21 (2), 274–contents. doi:10.1128/cmr.00045-07
Pang, P., Zheng, K., Wu, S., Xu, H., Deng, L., Shi, Y., et al. (2018). Baicalin Downregulates RLRs Signaling Pathway to Control Influenza A Virus Infection and Improve the Prognosis. Evid. Based Complement. Alternat Med. 2018, 4923062. doi:10.1155/2018/4923062
Peng, X. Q., He, Y., Zhou, H. F., Zhang, Y. Y., Yang, J. H., Chen, J. K., et al. (2015). Effect of Yinghua Pinggan Granule against Influenza A/H1N1 Virus In Vivo. Zhongguo Zhong Yao Za Zhi 40 (19), 3845–3850. doi:10.4268/cjcmm20151926
Peng, X. Q., Zhou, H. F., Lu, Y. Y., Chen, J. K., Wan, H. T., and Zhang, Y. Y. (2016). Protective Effects of Yinhuapinggan Granule on Mice with Influenza Viral Pneumonia. Int. Immunopharmacol 30, 85–93. doi:10.1016/j.intimp.2015.11.029
Peng, X. Q., Zhou, H. F., Zhang, Y. Y., Yang, J. H., Wan, H. T., and He, Y. (2016). Antiviral Effects of Yinhuapinggan Granule against Influenza Virus Infection in the ICR Mice Model. J. Nat. Med. 70 (1), 75–88. doi:10.1007/s11418-015-0939-z
Qu, X. Y., Li, Q. J., Zhang, H. M., Zhang, X. J., Shi, P. H., Zhang, X. J., et al. (2016). Protective Effects of Phillyrin against Influenza A Virus In Vivo. Arch. Pharm. Res. 39 (7), 998–1005. doi:10.1007/s12272-016-0775-z
Ren, Y., Yao, M. C., Huo, X. Q., Gu, Y., Zhu, W. X., Qiao, Y. J., et al. (2020). Study on Treatment of "cytokine Storm" by Anti-2019-nCoV Prescriptions Based on Arachidonic Acid Metabolic Pathway. Zhongguo Zhong Yao Za Zhi 45 (6), 1225–1231. doi:10.19540/j.cnki.cjcmm.20200224.405
Richard, S. A. (2021). Exploring the Pivotal Immunomodulatory and Anti-inflammatory Potentials of Glycyrrhizic and Glycyrrhetinic Acids. Mediators Inflamm. 2021, 6699560. doi:10.1155/2021/6699560
Rong, R., Li, R. R., Hou, Y. B., Li, J., Ding, J. X., Zhang, C. B., et al. (2016). Mahuang-Xixin-Fuzi Decoction Reduces the Infection of Influenza A Virus in Kidney-Yang Deficiency Syndrome Mice. J. Ethnopharmacol 192, 217–224. doi:10.1016/j.jep.2016.07.017
Runfeng, L., Yunlong, H., Jicheng, H., Weiqi, P., Qinhai, M., Yongxia, S., et al. (2020). Lianhuaqingwen Exerts Anti-viral and Anti-inflammatory Activity against Novel Coronavirus (SARS-CoV-2). Pharmacol. Res. 156, 104761. doi:10.1016/j.phrs.2020.104761
Schett, G., Sticherling, M., and Neurath, M. F. (2020). COVID-19: Risk for Cytokine Targeting in Chronic Inflammatory Diseases?. Nat. Rev. Immunol. 20 (5), 271–272. doi:10.1038/s41577-020-0312-7
Schwarz, S., Sauter, D., Wang, K., Zhang, R., Sun, B., Karioti, A., et al. (2014). Kaempferol Derivatives as Antiviral Drugs against the 3a Channel Protein of Coronavirus. Planta Med. 80 (2-3), 177–182. doi:10.1055/s-0033-1360277
Schwarz, S., Wang, K., Yu, W., Sun, B., and Schwarz, W. (2011). Emodin Inhibits Current through SARS-Associated Coronavirus 3a Protein. Antivir. Res 90 (1), 64–69. doi:10.1016/j.antiviral.2011.02.008
Segal, L. N., and Blaser, M. J. (2014). A Brave New World: the Lung Microbiota in an Era of Change. Ann. Am. Thorac. Soc. 11 Suppl 1 (Suppl. 1), S21–S27. doi:10.1513/AnnalsATS.201306-189MG
Shi, C. C., Zhu, H. Y., Li, H., Zeng, D. L., Shi, X. L., Zhang, Y. Y., et al. (2020). Regulating the Balance of Th17/Treg Cells in Gut-Lung axis Contributed to the Therapeutic Effect of Houttuynia Cordata Polysaccharides on H1N1-Induced Acute Lung Injury. Int. J. Biol. Macromol 158, 52–66. doi:10.1016/j.ijbiomac.2020.04.211
Shi, H., Ren, K., Lv, B., Zhang, W., Zhao, Y., Tan, R. X., et al. (2016). Baicalin from Scutellaria Baicalensis Blocks Respiratory Syncytial Virus (RSV) Infection and Reduces Inflammatory Cell Infiltration and Lung Injury in Mice. Sci. Rep. 6, 35851. doi:10.1038/srep35851
Shi, T., Ooi, Y., Zaw, E. M., Utjesanovic, N., Campbell, H., Cunningham, S., et al. (2020). Association between Respiratory Syncytial Virus-Associated Acute Lower Respiratory Infection in Early Life and Recurrent Wheeze and Asthma in Later Childhood. J. Infect. Dis. 222 (Suppl. 7), S628–s33. doi:10.1093/infdis/jiz311
Shi, X., Chen, G., Wei, J., Feng, D., Chen, Y., Zhou, H., et al. (2018). UHPLC-Q-TOF MS-Based Metabolic Analysis for the Therapeutic Efficacy of "Xuebijing Injection" against Sepsis-Induced Acute Lung Injury. Evid. Based Complement. Alternat Med. 2018, 8514619. doi:10.1155/2018/8514619
Shieh, W. J., Blau, D. M., Denison, A. M., Deleon-Carnes, M., Adem, P., Bhatnagar, J., et al. (2009). 2009 Pandemic Influenza A (H1N1): Pathology and Pathogenesis of 100 Fatal Cases in the United States. Am. J. Pathol. 177 (1), 166–175. doi:10.2353/ajpath.2010.100115
Sithisarn, P., Michaelis, M., Schubert-Zsilavecz, M., and Cinatl, J. (2013). Differential Antiviral and Anti-inflammatory Mechanisms of the Flavonoids Biochanin A and Baicalein in H5N1 Influenza A Virus-Infected Cells. Antivir. Res 97 (1), 41–48. doi:10.1016/j.antiviral.2012.10.004
Song, J., Han, Q., Qiao, C., Yip, Y., and Xu, H. (2007). Simultaneous Determination of Multiple Marker Constituents in Concentrated Gegen Tang Granule by High Performance Liquid Chromatography. Chin. Med. 2, 7. doi:10.1186/1749-8546-2-7
Song, J., Zhang, L., Xu, Y., Yang, D., Zhang, L., Yang, S., et al. (2021). The Comprehensive Study on the Therapeutic Effects of Baicalein for the Treatment of COVID-19 In Vivo and In Vitro. Biochem. Pharmacol. 183, 114302. doi:10.1016/j.bcp.2020.114302
Su, H. X., Yao, S., Zhao, W. F., Li, M. J., Liu, J., Shang, W. J., et al. (2020). Anti-SARS-CoV-2 Activities In Vitro of Shuanghuanglian Preparations and Bioactive Ingredients. Acta Pharmacol. Sin 41 (9), 1167–1177. doi:10.1038/s41401-020-0483-6
Sun, Q., Cao, H., Zhou, Y., Wang, X., Jiang, H., Gong, L., et al. (2016). Qualitative and Quantitative Analysis of the Chemical Constituents in Mahuang-Fuzi-Xixin Decoction Based on High Performance Liquid Chromatography Combined with Time-Of-Flight Mass Spectrometry and Triple Quadrupole Mass Spectrometers. Biomed. Chromatogr. 30 (11), 1820–1834. doi:10.1002/bmc.3758
Sun, Q. H., Zhang, J., Li, Z. Z., Du, B. X., Jiang, H. Q., Yang, Y., et al. (2017). Action Mechanism of Mahuang Xixin Fuzi Decoction for Mice with Influenza Based on Metabolomics Information]. Zhongguo Zhong Yao Za Zhi 42 (04), 763–771. doi:10.19540/j.cnki.cjcmm.20170103.022
Sun, Z., Zuo, L., Sun, T., Tang, J., Ding, D., Zhou, L., et al. (2017). Chemical Profiling and Quantification of XueBiJing Injection, a Systematic Quality Control Strategy Using UHPLC-Q Exactive Hybrid Quadrupole-Orbitrap High-Resolution Mass Spectrometry. Sci. Rep. 7 (1), 16921. doi:10.1038/s41598-017-17170-y
Swank, G. M., and Deitch, E. A. (1996). Role of the Gut in Multiple Organ Failure: Bacterial Translocation and Permeability Changes. World J. Surg. 20 (4), 411–417. doi:10.1007/s002689900065
Synowiec, A., Szczepański, A., Barreto-Duran, E., Lie, L. K., and Pyrc, K. (2021). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): a Systemic Infection. Clin. Microbiol. Rev. 34 (2). doi:10.1128/cmr.00133-20
Tao, Z., Chen, J., Su, J., Wu, S., Yuang, Y., Yao, H., et al. (2020). Quantitative Proteomics Analysis of Systemic Responses and Biological Mechanisms of ShuFengJieDu Capsule Using H1N1-Infected RAW264.7 Cells. ACS Omega 5 (25), 15417–15423. doi:10.1021/acsomega.0c01545
Tong, J. Y., Wong, A., Zhu, D., Fastenberg, J. H., and Tham, T. (2020). Author Reply to: “Comment on ‘The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-Analysis’”. Otolaryngol. Head Neck Surg. 163 (4), 853. doi:10.1177/019459982092647310.1177/0194599820934760
Umeoguaju, F. U., Ephraim-Emmanuel, B. C., Patrick-Iwuanyanwu, K. C., Zelikoff, J. T., and Orisakwe, O. E. (2021). Plant-Derived Food Grade Substances (PDFGS) Active against Respiratory Viruses: A Systematic Review of Non-clinical Studies. Front. Nutr. 8, 606782. doi:10.3389/fnut.2021.606782
Wang, C., Sun, S., and Ding, X. (2021). The Therapeutic Effects of Traditional Chinese Medicine on COVID-19: a Narrative Review. Int. J. Clin. Pharm. 43 (1), 35–45. doi:10.1007/s11096-020-01153-7
Wang, J., Li, F., Wei, H., Lian, Z. X., Sun, R., and Tian, Z. (2014). Respiratory Influenza Virus Infection Induces Intestinal Immune Injury via Microbiota-Mediated Th17 Cell-dependent Inflammation. J. Exp. Med. 211 (12), 2397–2410. doi:10.1084/jem.20140625
Wang, J., and Tian, Z. (2015). How Lung Infection Leads to Gut Injury. Oncotarget 6 (40), 42394–42395. doi:10.18632/oncotarget.6470
Wang, K., Yan, H., Wu, S., Wang, H., Li, Y., and Jiang, J. (2021). Inhibitory Effect of Qing-Fei-Pai-Du Decoction on Coronavirus In Vitro. Acta Pharmaceutica Sinica 56 (05), 1400–1408.
Wang, P., Zhao, C., Lu, F., Wu, T., Wei, K., Li, L., et al. (2020). Effect of Influenza A Virus Infection on Pulmonary flora and Chemokines CCL5 and CXCL10 in Mice and Intervention of Maxing Shigan Decoction. Chin. Traditional Herbal Drugs 51 (21), 5523–5537.
Wang, P., Zhao, C., Lu, F., Wu, T., Zhang, X., Chen, C., et al. (2021). Effects of Maxing Shigan Decoction on Intestinal flora and Chemokines CCL5 and CXCL10 in Mice Infected with Influenza Virus. Chin. Traditional Herbal Drugs 52 (01), 160–175.
Wang, S. X., Wang, Y., Lu, Y. B., Li, J. Y., Song, Y. J., Nyamgerelt, M., et al. (2020). Diagnosis and Treatment of Novel Coronavirus Pneumonia Based on the Theory of Traditional Chinese Medicine. J. Integr. Med. 18 (4), 275–283. doi:10.1016/j.joim.2020.04.001
Wang, Y. P., Guo, Y., Wen, P. S., Zhao, Z. Z., Xie, J., Yang, K., et al. (2020). Three Ingredients of Safflower Alleviate Acute Lung Injury and Inhibit NET Release Induced by Lipopolysaccharide. Mediators Inflamm. 2020, 2720369. doi:10.1155/2020/2720369
Wang, Z., Chen, W., Li, Y., Zhang, S., Lou, H., Lu, X., et al. (2021). Reduning Injection and its Effective Constituent Luteoloside Protect against Sepsis Partly via Inhibition of HMGB1/TLR4/NF-κB/MAPKs Signaling Pathways. J. Ethnopharmacol 270, 113783. doi:10.1016/j.jep.2021.113783
Weber, T. P., and Stilianakis, N. I. (2021). Fomites, Hands, and the Transmission of Respiratory Viruses. J. Occup. Environ. Hyg. 18 (1), 1–4. doi:10.1080/15459624.2020.1845343
Wolkerstorfer, A., Kurz, H., Bachhofner, N., and Szolar, O. H. (2009). Glycyrrhizin Inhibits Influenza A Virus Uptake into the Cell. Antivir. Res 83 (2), 171–178. doi:10.1016/j.antiviral.2009.04.012
Wu, M. S., Yen, H. R., Chang, C. W., Peng, T. Y., Hsieh, C. F., Chen, C. J., et al. (2011). Mechanism of Action of the Suppression of Influenza Virus Replication by Ko-Ken Tang through Inhibition of the Phosphatidylinositol 3-kinase/Akt Signaling Pathway and Viral RNP Nuclear export. J. Ethnopharmacol 134 (3), 614–623. doi:10.1016/j.jep.2011.01.005
Xing, Q. Q., Liu, L. W., Zhao, X., Lu, Y., Dong, Y. M., and Liang, Z. Q. (2019). Serum Proteomics Analysis Based on Label-free Revealed the Protective Effect of Chinese Herbal Formula Gu-Ben-Fang-Xiao. Biomed. Pharmacother. 119, 109390. doi:10.1016/j.biopha.2019.109390
Xu, H., He, L., Chen, J., Hou, X., Fan, F., Wu, H., et al. (2019). Different Types of Effective Fractions from Radix Isatidis Revealed a Multiple-Target Synergy Effect against Respiratory Syncytial Virus through RIG-I and MDA5 Signaling Pathways, a Pilot Study to Testify the Theory of Superposition of Traditional Chinese Medicine Efficacy. J. Ethnopharmacol 239, 111901. doi:10.1016/j.jep.2019.111901
Xu, T., Zhou, L., Shi, Y., Liu, L., Zuo, L., Jia, Q., et al. (2018). Metabolomics Approach in Lung Tissue of Septic Rats and the Interventional Effects of Xuebijing Injection Using UHPLC-Q-Orbitrap-HRMS. J. Biochem. 164 (6), 427–435. doi:10.1093/jb/mvy070
Yang, C., Wang, Y., He, J., Yan, W., Jiang, H., Chen, Q., et al. (2020). Lianhua-Qingwen Displays Antiviral and Anti-inflammatory Activity and Synergistic Effects with Oseltamivir against Influenza B Virus Infection in the Mouse Model. Evid. Based Complement. Alternat Med. 2020, 3196375. doi:10.1155/2020/3196375
Yang, Z. N., Sun, Y. M., Luo, S. Q., Chen, J. W., Chen, J. W., Yu, Z. W., et al. (2014). Quality Evaluation of Houttuynia Cordata Thunb. By High Performance Liquid Chromatography with Photodiode-Array Detection (HPLC-DAD). Pak J. Pharm. Sci. 27 (2), 223–231.
You, Y. N., Xing, Q. Q., Zhao, X., Ji, J. J., Yan, H., Zhou, T., et al. (2021). Gu-Ben-Fang-Xiao Decoction Modulates Lipid Metabolism by Activating the AMPK Pathway in Asthma Remission. Biomed. Pharmacother. 138, 111403. doi:10.1016/j.biopha.2021.111403
Yu, S., Zhu, Y., Xu, J., Yao, G., Zhang, P., Wang, M., et al. (2021). Glycyrrhizic Acid Exerts Inhibitory Activity against the Spike Protein of SARS-CoV-2. Phytomedicine 85, 153364. doi:10.1016/j.phymed.2020.153364
Zhang, Q., and Ye, M. (2009). Chemical Analysis of the Chinese Herbal Medicine Gan-Cao (Licorice). J. Chromatogr. A. 1216 (11), 1954–1969. doi:10.1016/j.chroma.2008.07.072
Zhang, Y., Ji, Z., and Han, D. (2019). Analysis on the Yin-Yang Theory of Shanghan Zabing Lun. China J. Traditional Chin. Med. Pharm. 34 (07), 2912–2917.
Zhang, Y., Li, Y., Wang, X., Qu, R., Li, J., Li, T., et al. (2020). Herbal Plants Coordinate COVID-19 in Multiple Dimensions - an Insight Analysis for Clinically Applied Remedies. Int. J. Med. Sci. 17 (18), 3125–3145. doi:10.7150/ijms.50260
Zhao, P., Yang, H. Z., Lv, H. Y., and Wei, Z. M. (2014). Efficacy of Lianhuaqingwen Capsule Compared with Oseltamivir for Influenza A Virus Infection: a Meta-Analysis of Randomized, Controlled Trials. Altern. Ther. Health Med. 20 (2), 25–30.
Zheng, S., Baak, J. P., Li, S., Xiao, W., Ren, H., Yang, H., et al. (2020). Network Pharmacology Analysis of the Therapeutic Mechanisms of the Traditional Chinese Herbal Formula Lian Hua Qing Wen in Corona Virus Disease 2019 (COVID-19), Gives Fundamental Support to the Clinical Use of LHQW. Phytomedicine 79, 153336. doi:10.1016/j.phymed.2020.153336
Zhi, H. J., Zhu, H. Y., Zhang, Y. Y., Lu, Y., Li, H., and Chen, D. F. (2019). In Vivo effect of Quantified Flavonoids-Enriched Extract of Scutellaria Baicalensis Root on Acute Lung Injury Induced by Influenza A Virus. Phytomedicine 57, 105–116. doi:10.1016/j.phymed.2018.12.009
Zhou, W., Lai, X., Wang, X., Yao, X., Wang, W., and Li, S. (2021). Network Pharmacology to Explore the Anti-inflammatory Mechanism of Xuebijing in the Treatment of Sepsis. Phytomedicine 85, 153543. doi:10.1016/j.phymed.2021.153543
Zhou, W., and Zhang, X. Y. (2013). Research Progress of Chinese Herbal Medicine Radix Isatidis (Banlangen). Am. J. Chin. Med. 41 (4), 743–764. doi:10.1142/s0192415x1350050x
Zhu, H., Lu, X., Ling, L., Li, H., Ou, Y., Shi, X., et al. (2018). Houttuynia Cordata Polysaccharides Ameliorate Pneumonia Severity and Intestinal Injury in Mice with Influenza Virus Infection. J. Ethnopharmacol 218, 90–99. doi:10.1016/j.jep.2018.02.016
Zhu, H., Wang, N., and Yang, A. (2021). Exploration on the Pathogenesis and Treatment of Mental Abnormalities in Epidemic Disease in the Ming and Qing Dynasties. China J. Traditional Chin. Med. Pharm. 36 (05), 2468–2471.
Zhu, H. Y., Han, L., Shi, X. L., Wang, B. L., Huang, H., Wang, X., et al. (2015). Baicalin Inhibits Autophagy Induced by Influenza A Virus H3N2. Antivir. Res 113, 62–70. doi:10.1016/j.antiviral.2014.11.003
Zou, L., He, G., Lu, F., Li, L., Zhang, B., Dai, B., et al. (2018). Effect of Maxing Shigan Tang on Protein Expression Level of MCP-1 in Lung Tissue and Colon Tissue Based on Influenza Virus Infection Lung Model. Chin. J. Exp. Traditional Med. Formulae 24 (05), 100–106.
 Bao-Hong Li
Bao-Hong Li Zhong-Yuan Li1,
Zhong-Yuan Li1,  Qing-Hua Cui
Qing-Hua Cui